Alnylam's Heart Drug Achieves Key Goal, Shares Surge
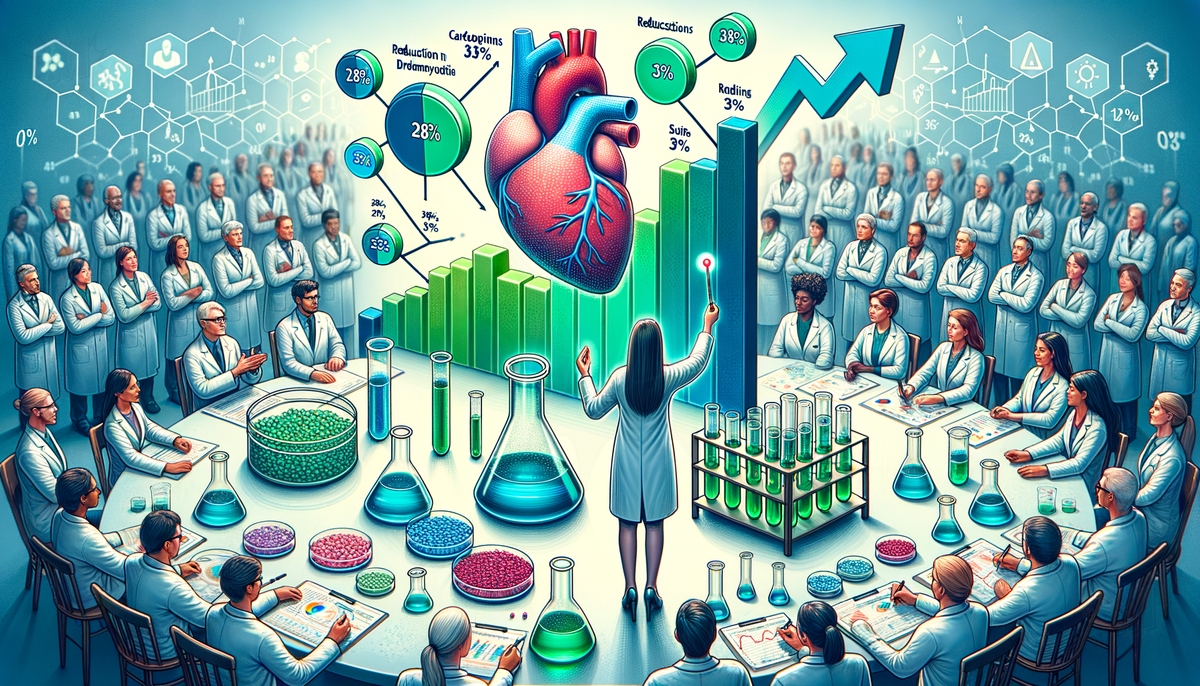
Alnylam Pharmaceuticals' drug, vutrisiran, has demonstrated positive results in a pivotal study, showing a reduction in the risk of death and recurrent cardiovascular complications by 28% in the overall trial population. This included patients who were already on standard-care treatment. In a subpopulation not on standard-treatment, the drug reduced risks by 33%. Additionally, in an open-label extension following the blinded period of the trial, vutrisiran reduced the chance of all-cause death by 36%.
The late-stage study of vutrisiran, aimed at treating ATTR amyloidosis with cardiomyopathy, met its main goal by significantly reducing deaths and recurrent cardiovascular events. The trial included 655 adults who took either vutrisiran or a placebo for up to three years. The company's shares saw a rise following the announcement of the results, and Alnylam plans to move forward with regulatory approval filing later this year. Alnylam also aims to expand the use of its top-selling product, Amvuttra, which is already approved for treating hereditary ATTR amyloidosis-related nerve damage in adults.